

The simplest or smallest amino acid is glycine. To repeat, the only difference in their structures is their R groups. Amino acid structureĢ0 amino acids make up all proteins. However, amino acids are different from each other based on the composition of their R groups. In each amino acid, an amino group and a carboxylic acid group attach to a carbon. They are important pieces of our bodies and assist in many processes such as protein synthesis. Topics Covered in Other ArticlesĪmino acids are the building blocks for polypeptides and proteins. It does not store any personal data.In this tutorial, you will learn about the 20 amino acid structures, along with their important biochemical properties. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary".

The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics".
These cookies ensure basic functionalities and security features of the website, anonymously.
Necessary cookies are absolutely essential for the website to function properly.
Structure of an amino acid free#
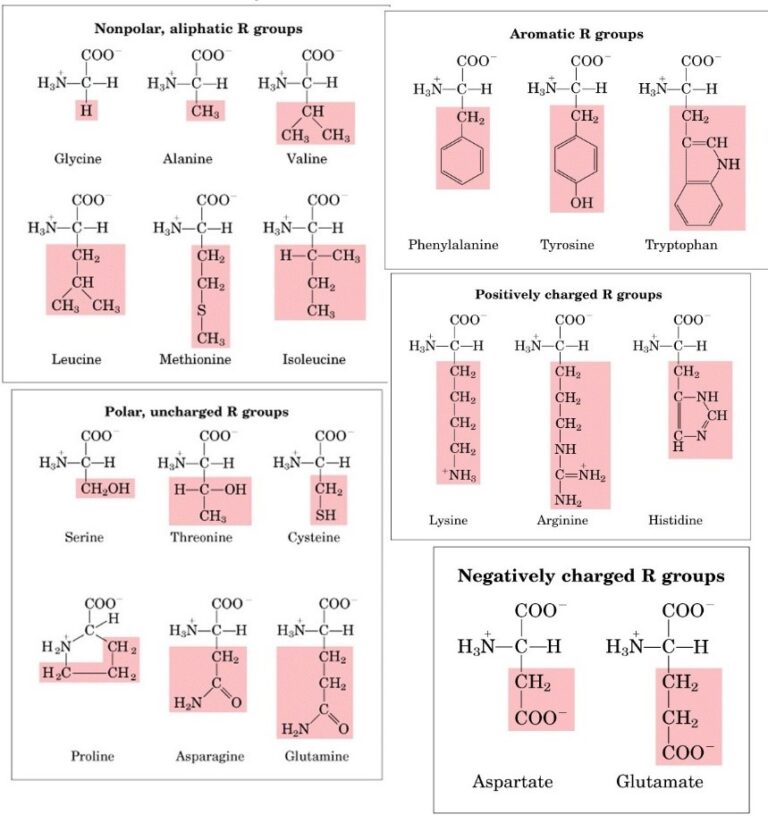
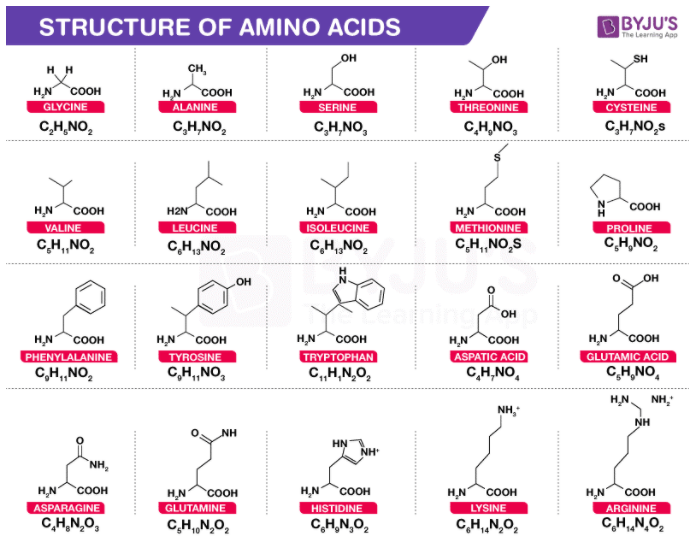
They occur both in the free state as well as in combined state, but not in proteins. Non-protein amino acids are many in number. Changes are made for amino acid derivatives like Asn for Asparagine (an amide). Amino acid names are abbreviated with the help of first three letters of the name. Rare amino acids are derived from the coded ones through modifications, e.g., hydroxyproline from proline, hydroxylysine from lysine (both in collagen). Incorporation of protein amino acids is controlled by triplet codes of DNA/mRNA.Ī protein may also possess non-coded amino acids. Twenty types of amino acids and amides occur in proteins (Table 9.3). Certain non-protein amino acids are dextrorotatory. Proline and hydroxyproline are called heterocyclic amino acids.Įxcept glycine, a-carbon is asymmetric and the amino acids are generally laevorotatory. In proline and hydroxyproline, amino group (-NH 2) is replaced by imino group (>NH) which also represents the tail of R-group. The hydrocarbon may further be polar (e.g., serine, glutamate or glutamic acid) or nonpolar (e.g., alanine). In others it may be straight or branched hydrocarbon chain or a cyclic group. R is represented by H in glycine, methyl group in alanine, hydroxymethyl in serine. Amino acids are, therefore, substituted methane’s where the four substituent groups occupy the four valency positions. The a-carbon also bears a variable hydrocarbon or alkyl group R and hydrogen. They are organic acids (with carboxylic group -COOH) having amino group (-NH 2) generally attached to a-carbon or carbon next to the carboxylic group.Ĭarboxylic group provides an acidic property to the amino acid while amino group gives it a basic reaction. The below mentioned article provides a study note on the Amino Acids.


 0 kommentar(er)
0 kommentar(er)
